However researchers don’t yet have a ton of familiarity with it, a recently portrayed process called erebosis could have significant ramifications for how the stomach keeps up with itself.
Consistently, billions of our cells pass on and new, sound ones have their spot. In a sound stomach lining, as in many tissues, a sort of cell passing called apoptosis is remembered to intervene this cycle on the whole all alone. In any case, scientists from RIKEN in Kobe, Japan, suspect they have found another sort of cell passing in the stomach of a natural product fly. The new cycle, which they call erebosis or “profound obscurity,” might be available in different tissues, the group reports April 25 in PLOS Biology — and assuming found in people, it could influence how we grasp sicknesses of the gastrointestinal lot.
María Domínguez Castellano, a neuroscientist at the Institute of Neurosciences in Alicante, Spain who didn’t deal with the review, tells The Scientist she tracked down the paper “extremely captivating,” and desires to see more from the specialists about the new putative type of cell passing. “They’ve obviously accomplished such a lot of work yet . . . [Erebosis] is plainly there and it’s captivating to such an extent that you need more.”
Unintentionally revealing an option in contrast to apoptosis
“The narrow minded thought is apoptosis is engaged with cell turnover in the stomach,” concentrate on coauthor Sa Kan Yoo, a researcher at RIKEN, tells The Scientist. He says that this doctrine is solid to such an extent that he was at first impervious to the possibility that he was checking out at another type of cell death.
The venture started quite a long while back, when Yoo and RIKEN PhD understudy Hannah Ciesielski were looking for a component that controls the foundational impacts of disease. A protein called Ance — the natural product fly type of the human angiotensin-changing over chemical, which is engaged with the guideline of pulse and electrolyte balance in people — was a top competitor. The scientists started to plan the areas of Ance in tissues all through the body of the organic product fly Drosophila melanogaster utilizing an immunizer that marks the protein with Green Fluorescent Protein (GFP) and that carried them to the stomach.
Their imaging tests uncovered that some stomach cells are brimming with Ance, while others produce very little. Interested, they chose to figure out why.
Yoo and his group thought they had staggered on another phone type that unequivocally communicated Ance, which they chose to call Ance cells. These Ance cells “were exceptionally strange,” says Yoo. At the point when the group watched Ance cells, they saw that many began to lose proteins, organelles, and significant particles they need to make due. ATP creation eased back. Their cores expanded, then leveled, and at last vanished. The cells additionally began losing their GFP, shining less and less splendidly. The way that the phones seemed to lose fluorescence over the long haul indicated to the scientists that the Ance cells were “extremely unique,” and should get going through a time of progress. They before long came to feel that the phones may be approaching the finish of their lives.
1,000,000 (or if nothing else three) ways of dieing in the stomach
All cells have a restricted life expectancy, and their passing can occur in more ways than one. As they age and aggregate transformations, inner or outer signs trigger apoptosis, which can be considered a coordinated auto-destruct. The phone psychologists and breaks up into discrete bundles called apoptotic bodies, which are subsequently consumed by cell-eating safe cells called phagocytes. Less usually, harmed, oxygen-starved, or carcinogenic cells can go through corruption, enlarging and ultimately blasting open to spill their items into the body. Cells can likewise pass on through autophagy, an interaction likened to eating themselves, which is believed to be achieved by an absence of food. In autophagy, cells disintegrate their interior items through autophagosomes, huge vesicles that stall the cell’s items.
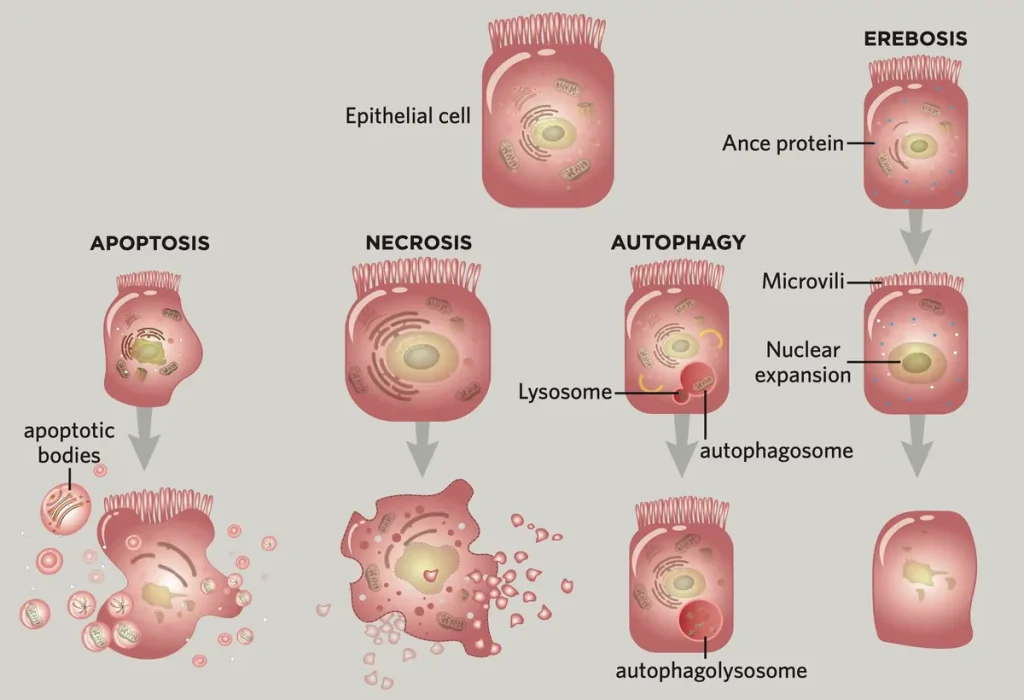
By then, Yoo and his group were all the while attempting to make sense of Ance cell movement inside the setting different types of cell demise, particularly apoptosis, as it is believed to be the most well-known driver of the stomach’s speedy (when at regular intervals to three-week) tissue turnover. They started looking for proof that Ance cells were creating markers of corruption and autophagy, the other, more uncommon types of cell passing. In any case, they neglected to find proof that any of the three were occurring. Moreover, inactivating caspases (which are atoms regularly found in cells going through apoptosis that signal cells to begin separating) with microRNAs neglected to prevent the phones from losing organelles, proteins, or ATP.
To sort out what was happening, the scientists utilized an overall cell passing marker called TUNEL, which names divided DNA. TUNEL named some Ance cells yet not others. The phones that were marked had lower GFP signs and vagrant cores, which unequivocally demonstrated that these phones were for sure moving toward the finish of their lives.
The analysts additionally took a gander at whether this recently portrayed, Ance-related pathway to death actually happened in Drosophila freaks that needed significant apoptosis, putrefaction, and autophagy-related proteins. In all cases, erebosis endured. On the whole, their discoveries highlighted one decision: Ance was a marker for a phone’s inevitable destiny — a sort of cell demise nobody had depicted previously, which they chose to call erebosis.
Erebosis: a bigger number of inquiries than responds to
That’s what yoo recognizes, in fact, the group didn’t demonstrate that cells are biting the dust through erebosis, nor have they ironed out a ton of the subtleties. However they’ve recorded that these phones are going through a cycle that appears to be hard to return from, they haven’t shown them vanishing continuously. They might in any case be alive, Yoo assumes, existing endlessly in a, extraordinary failure metabolic state. Likewise, precisely how erebotic cells start to lose organelles or separate cytoplasmic proteins is as yet unclear. “It’s truly difficult to demonstrate that a cell is biting the dust,” says Yoo, “It’s nearly . . . a philosophical inquiry.” But without organelles or a core, says Yoo, it just checks out that passing is not too far off for these cells.
“It’s as yet hazy how [erebosis] squeezes into homeostasis . . . furthermore, I need to find out about what other place erebosis is occurring,” says Dominguez.
Assuming that erebosis is a passing pathway, it could assist with making sense of confounding outcomes from different examinations, says Andreas Bergmann, a researcher the University of Massachusetts Medical School not associated with the review who composed a viewpoint on the paper that was distributed April 26 in PLOS Biology. “I was truly energized when I saw this work,” Bergmann expresses, concerning years, his lab experiences experienced issues showing apoptosis “with standard apoptotic markers.” And in a few past examinations, hindering apoptosis in stomach cells has eased back cell turnover, while in others, it didn’t. This shows that some other component might be involved.
The discoveries could likewise have clinical ramifications. According to faulty cell turnover, Yoo, is connected with a few gastrointestinal illnesses, including ulcerative colitis and gastroenteritis. On the off chance that erebosis happens in the human stomach, it could turn out badly and assume a part in specific sicknesses. Presently, Yoo is chipping away at understanding which qualities and proteins are engaged with erebosis, while his teammates are checking in the event that this cycle exists in well evolved creatures and in other Drosophila tissues.
What’s more, in an unusual wind, the scientists have previously observed that Ance isn’t really needed. The course of particle and organelle-unloading and cores smoothing proceeded with unabated when Ance was taken out utilizing miRNAs. Thus, despite the fact that stomach cells will generally take up Ance during erebosis, the specialists don’t yet have the foggiest idea why.
The significance or scarcity in that department of Ance isn’t the just erebosis secret still needing to be tackled. Both Bergmann and Dominguez Castellano express that there’s significantly more examining to do. For example, they’re anxious to get more familiar with what qualities and proteins control erebosis and what different tissues it could happen in. “This [story] isn’t only one paper,” Bergmann says, “It’s ten papers.”